Translational research stands as one of the most critical—and most challenging—phases of the pharmaceutical development journey. While basic research generates promising targets and clinical trials validate therapeutic efficacy, the translational phase determines which molecules advance, which indications to pursue, and ultimately, which programs will deliver value to patients and shareholders. Yet this crucial bridge between discovery and development remains one of the most time-consuming, resource-intensive, and risk-laden stages in the pharmaceutical pipeline.
The statistics are sobering. A typical drug takes 10-15 years and $2.6 billion to reach approval, with translational research consuming 30-40% of this timeline and budget. Worse, 90% of candidates that enter clinical development fail, often due to decisions made—or delayed—during the translational phase. Target validation proves insufficient. PK/PD models fail to predict human response. Safety signals emerge too late. Go/no-go decisions lack comprehensive evidence synthesis. Companion diagnostic strategies remain afterthoughts.
For pharmaceutical executives and investors, this translational bottleneck represents both a competitive vulnerability and an opportunity. Organizations that can compress translational timelines while improving decision quality gain years of market exclusivity, reduce capital at risk, and increase portfolio success rates.
We propose that K-Dense is a versatile, future-proof solution to these challenges: an agentic AI co-scientist platform purpose-built to transform translational research from a sequential, labor-intensive process into an integrated, insight-driven engine for drug development.
The Translational Research Challenge
The paradox at the heart of translational research is that while we generate more biological data than ever before—genomics, proteomics, patient-derived models, high-content imaging, electronic health records—our ability to synthesize this information into actionable decisions has not kept pace. A translational scientist evaluating a potential target must now integrate evidence from dozens of databases, hundreds of publications, multiple experimental modalities, and complex computational predictions. The sheer volume of data has become a liability rather than an asset.
Each year, over 1.5 million new research articles are published. Genetic databases expand by terabytes. Patient registries grow exponentially. Yet the human capacity to read, synthesize, and apply this knowledge remains fundamentally constrained. The average researcher can review perhaps 50-100 papers comprehensively. A translational team might synthesize evidence from 200-300 sources over several months. Meanwhile, the complete evidence base for a single target spans thousands of publications, dozens of databases, and terabytes of experimental data.
Consider the journey of a single target from identification to IND filing. A team must validate target expression across disease subtypes, assess druggability through structural analysis, evaluate competing mechanisms, model pharmacological interventions, predict human PK parameters, design safety studies informed by pathway biology, and develop biomarker strategies aligned with regulatory requirements. Each step involves specialized expertise, proprietary tools, and weeks of analysis. Sequential handoffs between functional groups introduce delays and information loss.
Traditional approaches rely on human experts performing these syntheses manually, supported by disconnected software tools. An excellent team executes this workflow in 12-18 months. A typical team takes 24-36 months. Meanwhile, competitive intelligence suggests another organization may be advancing a similar program.
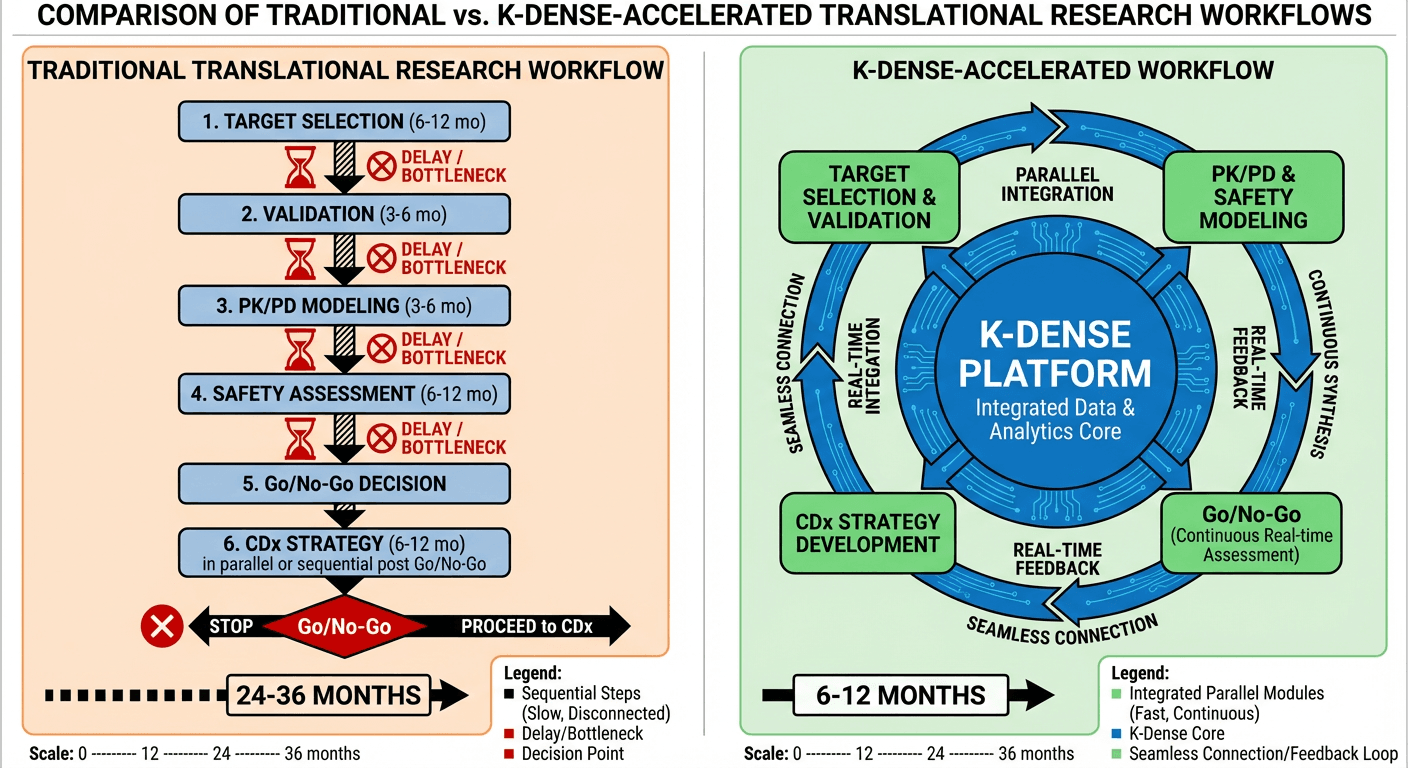
K-Dense fundamentally reimagines this process. Rather than a relay race where each expert completes their analysis before passing to the next, K-Dense enables parallel, integrated evidence generation with continuous synthesis across all translational workstreams (Figure 1). The result: translational timelines compressed by weeks or even months, decision quality improved through comprehensive evidence integration, and organizational bandwidth freed for strategic priorities.
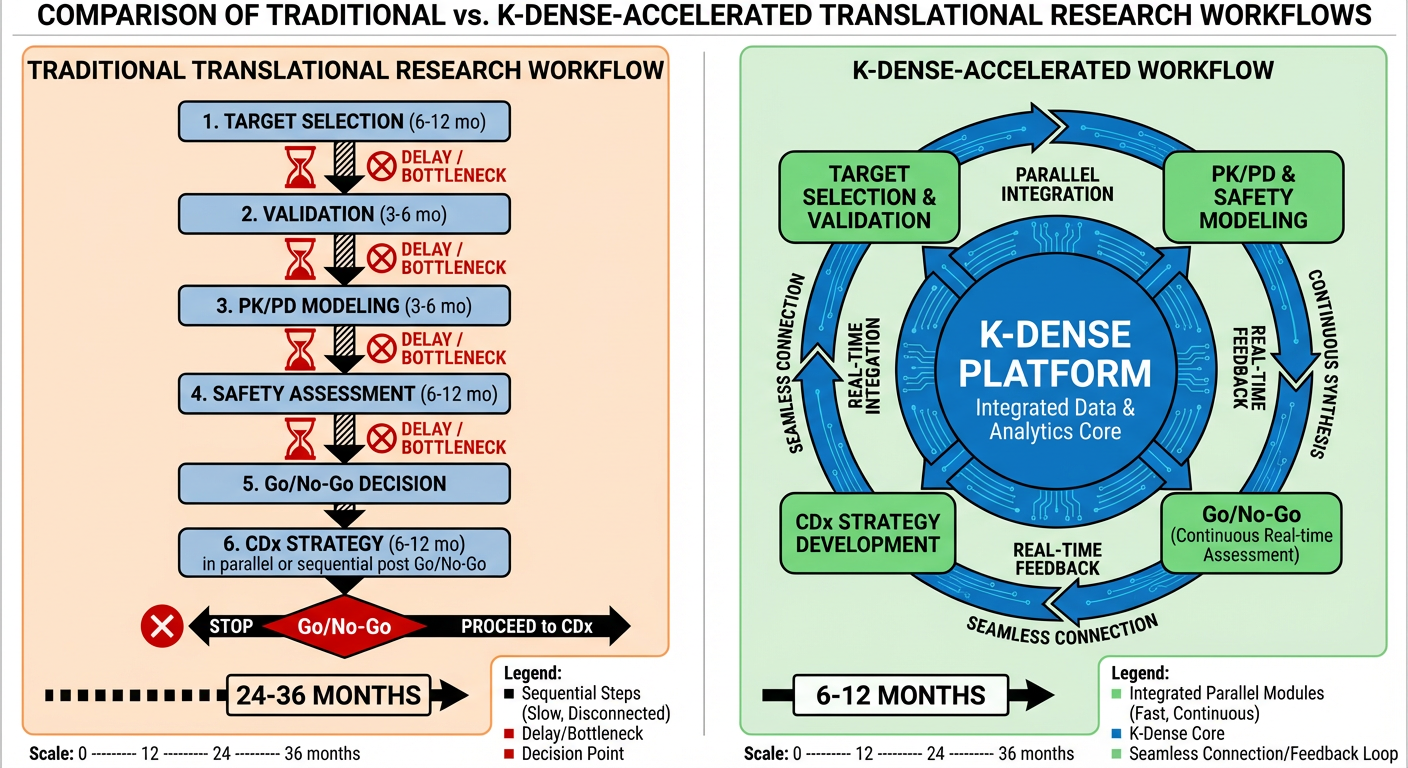
Figure 1: Traditional vs K-Dense-Accelerated Translational Research Workflow
Target and Indication Selection: Evidence-Based Prioritization
Target selection represents perhaps the most consequential decision in drug development. Choose well, and you build on validated biology with clear paths to clinical proof of concept. Choose poorly, and years of investment culminate in expensive failure.
Traditional target validation combines literature review, genetic evidence analysis, pathway mapping, competitive landscape assessment, and druggability evaluation—each performed by different specialists using different tools. The process is slow, subjective, and prone to confirmation bias. Critical evidence may be missed.
Agentic systems such as K-Dense will transform target selection through autonomous, multi-dimensional evidence synthesis, simultaneously:
- Analyzing genetic association data across hundreds of thousands of patients from UK Biobank, FinnGen, and disease-specific cohorts
- Mining millions of publications to identify mechanistic links, phenotypic associations, and translational precedents
- Evaluating protein structures and small molecule binding potential using state-of-the-art computational chemistry
- Mapping pathway connectivity to predict on-target effects and potential liabilities
- Assessing competitive landscape including clinical trials, patent filings, and corporate disclosures
- Integrating tissue expression patterns, disease subtype stratification, and biomarker opportunities
This comprehensive analysis (Figure 2), which would require months from multiple specialists, completes in hours. More importantly, K-Dense can generate an integrated evidence report that quantifies confidence across multiple dimensions: genetic support, mechanistic understanding, druggability, competitive position, and commercial potential.
For indication selection, K-Dense extends this framework to evaluate multiple disease contexts simultaneously. A target with modest genetic support in one indication may show stronger validation in a related condition. K-Dense identifies these strategic opportunities through parallel, comprehensive evidence synthesis that human teams would require weeks to perform manually.
The impact of agentic AI on portfolio strategy will be profound. Rather than sequential target evaluation leading to conservative selections, K-Dense and similar systems enable rapid assessment of multiple targets across multiple indications, empowering leadership to make bold, evidence-based bets on differentiated opportunities.
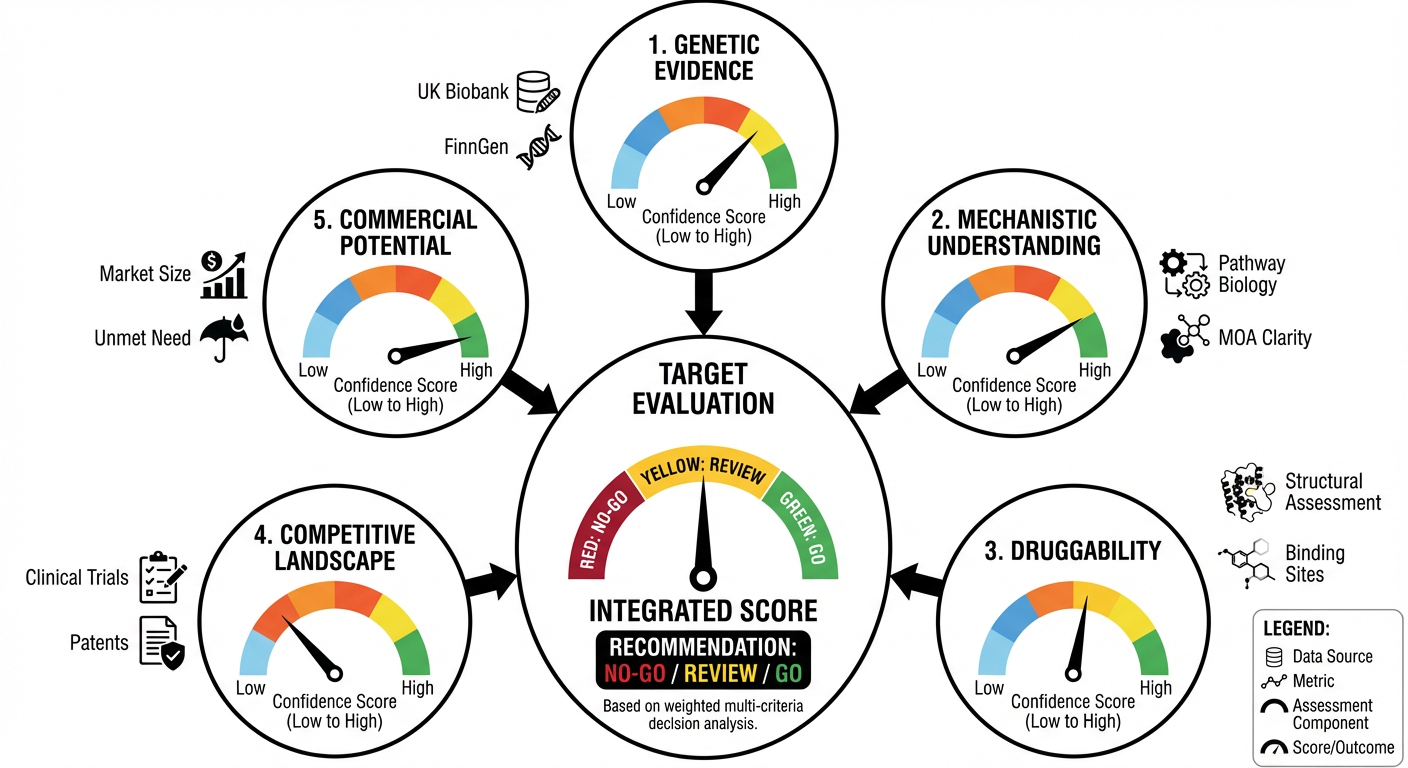
Figure 2: Multi-Criteria Target Selection Framework
PK/PD Modeling: From Preclinical Data to Human Prediction
Pharmacokinetic and pharmacodynamic modeling represents the quantitative heart of translational research. Accurate PK/PD models enable rational dose selection, predict therapeutic windows, guide formulation strategy, and provide the exposure-response framework essential for clinical development.
K-Dense accelerates PK/PD modeling through AI-powered integration of preclinical data, physiologically-based modeling, and translational scaling (Figure 3). The platform ingests data from in vitro metabolism studies, preclinical PK experiments across species, and target engagement assays, then automatically constructs population PK/PD models that predict human exposure and response.
An example workflow would include:
Absorption Prediction: Integration of solubility, permeability, and formulation parameters to predict oral bioavailability using machine learning trained on thousands of clinical compounds.
Distribution Modeling: Physiologically-based compartmental models incorporating tissue binding, protein binding, and transporters to predict volume of distribution and tissue exposure.
Metabolism & Clearance: Analysis of in vitro CYP data, hepatocyte stability, and preclinical clearance to predict human elimination pathways using allometric scaling refined by AI.
Target Engagement: PK/PD modeling that links systemic exposure to target occupancy and downstream pharmacology, enabling prediction of efficacious doses in humans.
Population Variability: Simulation of inter-individual variability in exposure and response across diverse populations, informing dose selection and clinical trial design.
Most critically, K-Dense can continuously refine these predictions as new data emerges. Initial models based on in vitro and animal data are automatically updated when formulation studies complete. This iterative refinement ensures that IND-enabling decisions are informed by the most current and comprehensive PK/PD understanding.
For pharmaceutical executives, this capability translates directly to reduced risk and accelerated timelines. Rather than waiting for sequential PK studies before initiating modeling, agentic AI enables continuous, parallel analysis. While current tools like NONMEM and Simcyp are the current regulatory gold standards, they require significant manual effort to operate. Where a NONMEM user must manually write control streams and guess initial parameters, K-Dense can autonomously:
- Scan data for structure and anomalies.
- Select appropriate models based on the data signature.
- Estimate parameters using AI-driven initialization to prevent convergence failures.
- Scale computation to the cloud instantly.
K-Dense does not replace the underlying mathematics of PK/PD, but replaces the manual labor required to execute them, offering a modern, Python-first alternative to the rigid, proprietary interfaces of legacy software.
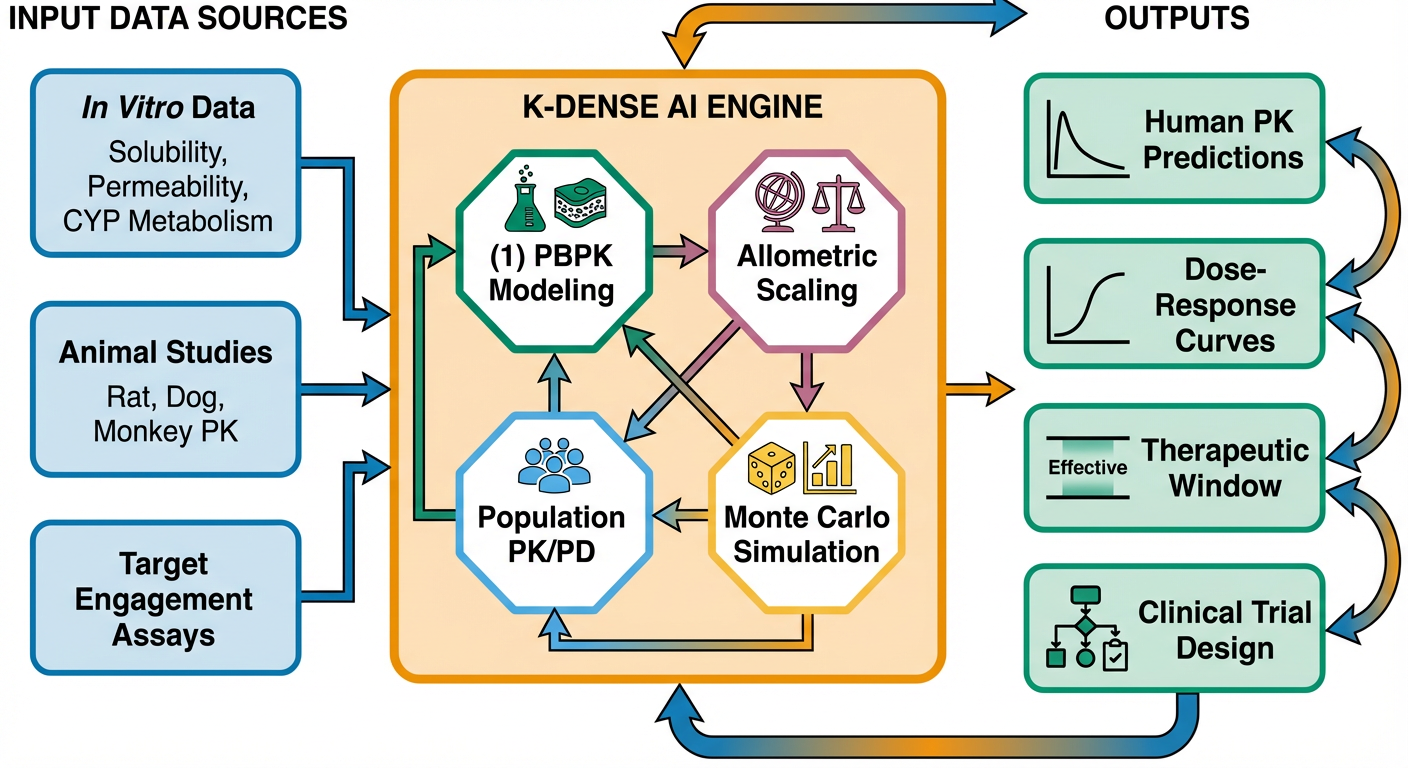
Figure 3: Integrated PK/PD Modeling Pipeline
Safety Assessment: Comprehensive Risk Evaluation
Safety failures remain the leading cause of clinical attrition, with many toxicities predictable from preclinical data if comprehensively analyzed. Traditional safety assessment involves disconnected evaluation of in vitro toxicity screens, animal toxicology studies, and human safety databases, with synthesis occurring late in development.
K-Dense can integrate safety assessment throughout the translational process by simultaneously evaluating:
Target-Based Liabilities: Pathway analysis to predict on-target toxicities based on tissue expression, physiological functions, and genetic knockout phenotypes.
Off-Target Risks: Computational prediction of binding to anti-targets including ion channels, GPCRs, and kinases associated with clinical toxicities.
DMPK-Related Toxicity: Prediction of reactive metabolite formation, transporter-mediated drug interactions, and accumulation in safety-relevant tissues.
Translational Toxicology: Integration of preclinical toxicity findings with human genetic evidence and adverse event databases to assess human relevance.
Biomarker Strategy: Identification of translational biomarkers that enable early detection of toxicity in clinical trials.
This integrated approach enables safety-conscious decision-making at every stage of development. Rather than discovering liabilities at costly late-stage milestones, K-Dense surfaces potential risks when intervention is feasible and inexpensive.
Go/No-Go Decisions: Evidence-Integrated Program Management
Development decisions are rarely straightforward. The question is rarely "should we advance this program?" but rather "given everything we know about target biology, competitive landscape, commercial opportunity, and organizational priorities, how should we optimize this program's path forward?" This strategic integration of scientific evidence with business context separates exceptional pharmaceutical organizations from average ones.
K-Dense transforms go/no-go decisions from intuition-based milestones to evidence-integrated assessments (Figure 4). For each decision point, the platform synthesizes:
Scientific Evidence: Comprehensive evaluation of target validation, translational confidence, and remaining scientific risk.
Clinical Positioning: Analysis of competitive trials, emerging clinical data, and potential differentiation strategies.
Regulatory Context: Assessment of regulatory precedents, evolving guidance, and pathway optimization opportunities.
Commercial Outlook: Integration of market dynamics, pricing expectations, and commercial scenarios.
Portfolio Context: Comparison with other programs competing for organizational resources and strategic alignment with corporate priorities.
This evidence integration replaces the traditional approach where committees make consequential decisions based on incomplete information synthesized under time pressure. K-Dense ensures that every decision point is informed by the most comprehensive, current evidence available.
The result is faster decisions with higher quality. Programs that should advance move forward with confidence. Programs with fundamental issues are identified earlier when pivot costs are lower. Most importantly, leadership can allocate capital more efficiently.
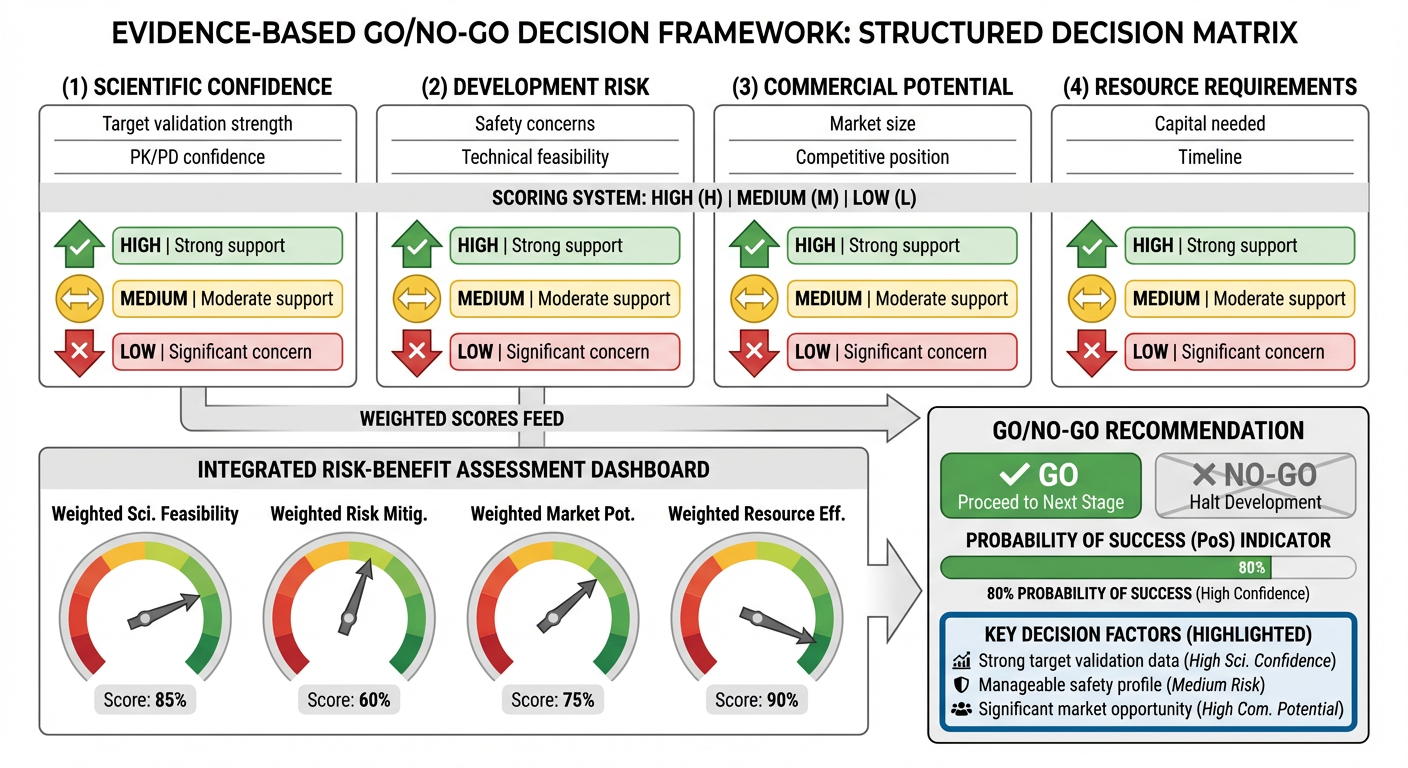
Figure 4: Evidence-Based Go/No-Go Decision Framework
Diagnostics and Companion Diagnostics: Integrated Development
The era of one-size-fits-all therapeutics is ending. Precision medicine—enabled by biomarkers and companion diagnostics—promises to improve clinical outcomes while de-risking development by enriching trial populations. Yet companion diagnostic (CDx) development often lags therapeutic development, treated as an afterthought.
This disconnect creates substantial risk. CDx strategies defined late may prove technically infeasible or regulatorily unacceptable. Patient stratification biomarkers identified during Phase 2 require costly assay development that delays pivotal trials.
K-Dense can be directed to embed CDx strategy into translational research from day one. The platform can evaluate potential biomarkers across the entire development timeline:
Mechanism-Based Biomarkers: Identification of biomarkers directly linked to target engagement or pathway modulation, enabling early proof of mechanism studies.
Patient Selection Biomarkers: Analysis of genetic, proteomic, and imaging biomarkers that predict response, enabling enrichment strategies that improve trial success probability.
Pharmacodynamic Biomarkers: Selection of translational biomarkers that enable PK/PD modeling and dose optimization across preclinical and clinical development.
Safety Monitoring Biomarkers: Identification of early indicators of toxicity that enable adaptive trial designs with pre-specified safety monitoring.
Commercial CDx Strategy: Evaluation of technical feasibility, regulatory pathway, and partnership options for companion diagnostics that enable market access.
For each biomarker category, K-Dense can assess technical feasibility, regulatory acceptability, and operational complexity. This comprehensive analysis enables leadership to make informed decisions about biomarker strategy early in development.
The impact of integrated agentic AI on development timelines and clinical success will be substantial (Figure 5). Programs with integrated CDx strategies from inception complete development 12-18 months faster. Clinical success rates improve by 20-30% when patient selection biomarkers enable enrichment.
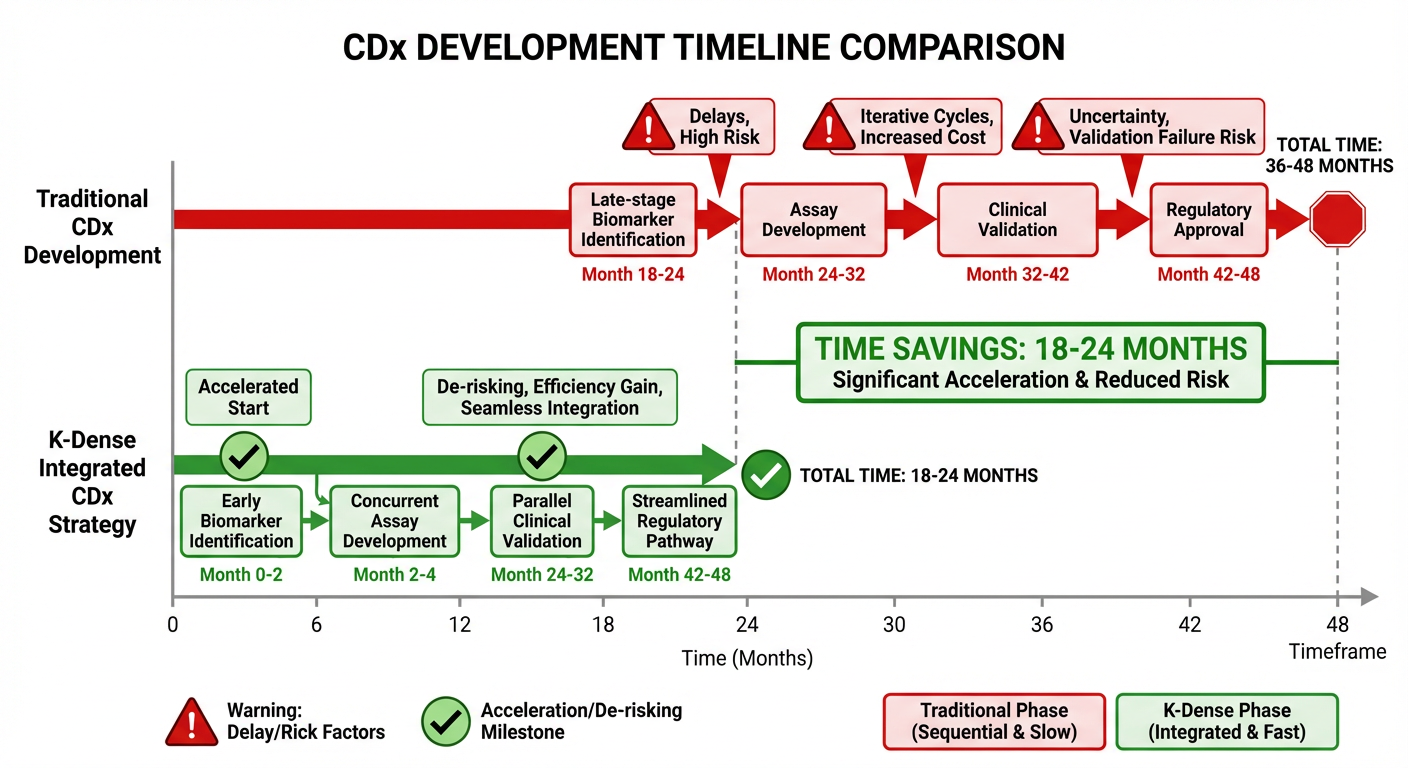
Figure 5: Accelerated CDx Development Timeline
The Compounding Advantage: Integration Across the Pipeline
While each capability delivers value independently, agentic AI's true power will emerge from integration across the entire translational pipeline.
In traditional workflows, each workstream operates largely independently. Integration occurs episodically, often at formal decision gates, with limited feedback loops between functions.
K-Dense enables continuous, bidirectional integration. PK/PD predictions inform safety study design by identifying relevant exposure ranges. Safety findings refine target product profiles, which update competitive assessments. Biomarker availability influences clinical trial design, which feeds back to inform IND-enabling study selection. Every analysis informs every other analysis.
This integration delivers several compounding advantages:
Reduced Iteration Cycles: Rather than sequential cycles that take months, K-Dense enables continuous refinement with weekly or daily updates as new data emerges.
Improved Consistency: Evidence synthesis follows consistent methodologies across programs, enabling valid comparisons and portfolio-level optimization.
Organizational Learning: Insights from completed programs automatically improve future analyses through continuously refined models.
Reduced Dependencies: Domain expertise is captured in K-Dense's analytical frameworks, reducing vulnerability to key person dependencies and enabling scaling.
For pharmaceutical executives evaluating translational research capabilities, this integration represents the fundamental advantage. Point solutions that address individual pain points deliver marginal improvements. Integrated agentic platforms like K-Dense can deliver transformative acceleration.
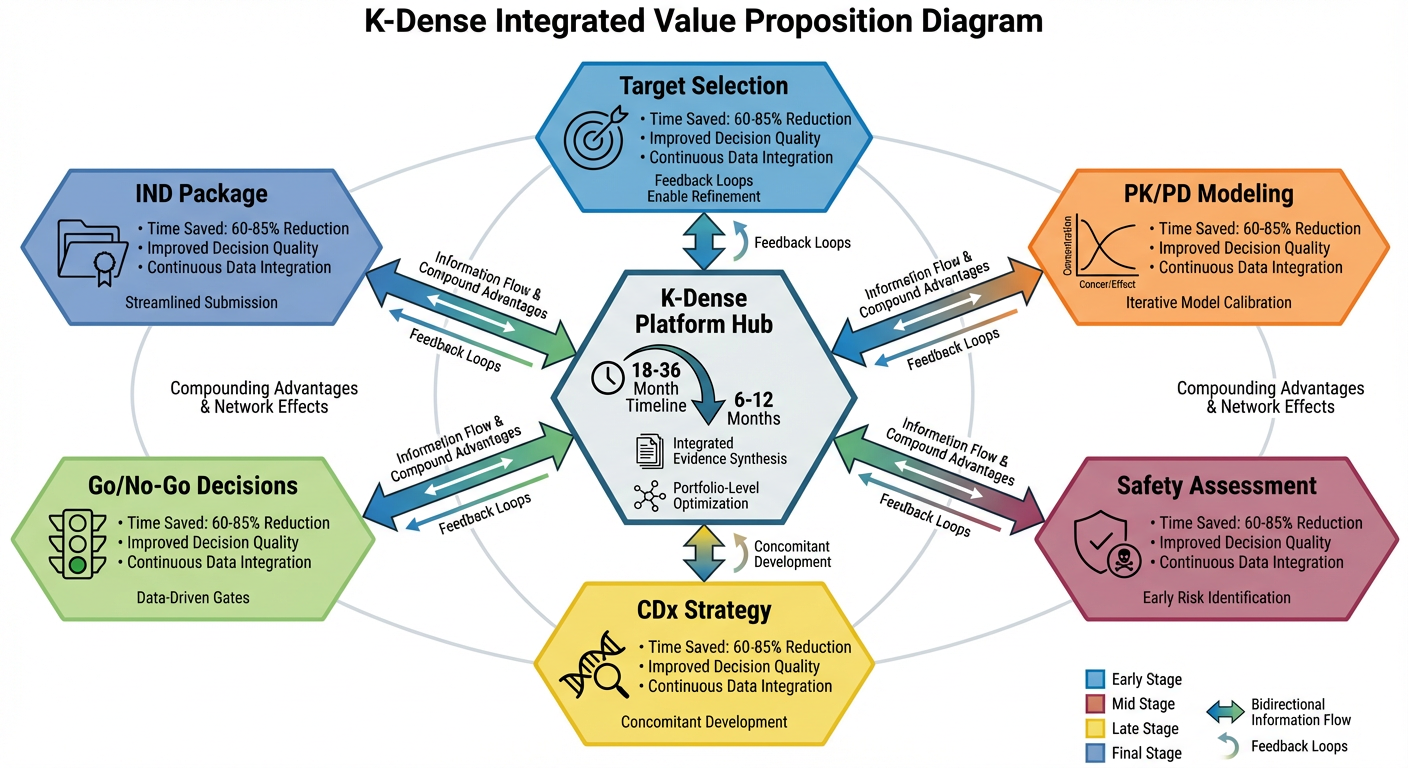
Figure 6: K-Dense Value Proposition – Integrated Acceleration
Quantifying the Agentic AI Advantage
The strategic case for K-Dense rests on quantifiable acceleration across the translational pipeline: For a typical drug with projected peak sales of $1 billion, one year of accelerated approval translates to approximately $600-800 million in additional net present value. For a blockbuster with $5 billion peak sales, the value exceeds $3 billion.
Beyond timeline acceleration, K-Dense improves decision quality in ways equally valuable. Better target selection reduces clinical attrition. Superior PK/PD predictions enable optimal dose selection. Comprehensive safety assessment prevents late-stage toxicity failures. Integrated CDx strategies improve patient selection and commercial positioning.
The Future of Translational Research
The introduction of agentic AI systems such as K-Dense into pharmaceutical translational research will produce more than incremental improvement—it is a paradigm shift in how organizations conduct evidence synthesis and make decisions.
Traditional AI applications serve as assistants: tools that help human experts work faster. They accelerate literature searches, automate routine analyses, and generate preliminary hypotheses. The human expert remains the bottleneck, synthesizing information and drawing conclusions. These tools might improve individual productivity by 20-30%, but they don't fundamentally transform the process.
K-Dense operates as an executor: an autonomous agent that performs comprehensive analyses and generates actionable recommendations. It doesn't just find relevant papers—it reads them, extracts key evidence, synthesizes conclusions, and quantifies confidence across multiple dimensions. It doesn't just run PK/PD models—it integrates preclinical data, selects appropriate modeling approaches, generates predictions, quantifies uncertainty, and recommends optimal clinical strategies. It doesn't compile safety data—it performs comprehensive toxicity assessments, predicts human relevance, and recommends risk mitigation approaches.
This shift from assistant to executor unlocks step-function improvements in productivity. Human experts are freed from time-consuming synthesis tasks to focus on strategic thinking, experimental design, and high-stakes decision-making—the irreplaceable human contributions that create competitive advantage. Translational timelines could be compressed not by 20-30% but by 60-70%—the result of fundamental process transformation from agentic AI rather than incremental optimization.
For pharmaceutical leadership, this transition raises a critical strategic question: will your organization lead this transformation, or be disrupted by it? Early adopters are already experiencing competitive advantages in portfolio velocity, decision quality, and capital efficiency. Late adopters will find themselves racing against organizations operating at a fundamentally faster pace, with superior evidence synthesis and more confident decision-making at every stage of development.
The investment thesis is compelling. Organizations that comprehensively deploy agentic AI across their translational pipeline will accelerate programs by as much as 12-24 months, reduce development costs by as much as 30-40%, and potentially improve clinical success rates by 20-30% through better target selection and patient stratification. For a typical pharmaceutical portfolio, these improvements translate to billions of dollars in incremental value through accelerated approvals, reduced failures, and optimized capital allocation.
Getting Started
For pharmaceutical executives and investors seeking to understand how agentic AI can accelerate your translational research programs, K-Dense offers consultative engagements designed to demonstrate value using your actual programs and priorities.
A typical engagement includes:
Portfolio Assessment: Evaluation of your current translational pipeline to identify programs where K-Dense can deliver immediate impact.
Proof of Concept: Focused analysis on a selected program, demonstrating K-Dense's capabilities using your data and decision criteria.
Implementation Planning: Development of a roadmap for K-Dense deployment across your organization.
Value Quantification: Detailed financial modeling of expected impact on your portfolio, including timeline acceleration and probability-adjusted NPV improvement.
Take the Next Step
The translational research bottleneck is no longer inevitable. Organizations that embrace AI-powered evidence synthesis and decision support will translate laboratory insights into clinical reality faster, with higher success rates and greater capital efficiency.
K-Dense is purpose-built to deliver this transformation. Our platform combines comprehensive scientific capabilities, integrated workflows, and autonomous execution to accelerate translational research while improving decision quality at every stage.
Schedule a meeting with K-Dense to discuss options for incorporating K-Dense into your research workflows.
Contact brian.laffin@k-dense.ai or visit k-dense.ai/enterprise to begin the conversation.
The future of translational research is faster, smarter, and more successful. The organizations that get there first will define the competitive landscape for the decade ahead.
About K-Dense: K-Dense is the leading AI platform for scientific research and pharmaceutical development. Our technology enables autonomous execution of complex research workflows across target identification, translational research, clinical development, and regulatory strategy. Learn more at k-dense.ai.